Seronegative spondyloarthropathies & inflammatory low back pain - Part 2
When it comes to the diagnosis of low back pain, in up to 85% of cases, no clear diagnosis can be made and pathological changes are not closely related to symptoms (Javik & Deyo, 2002). While the exact prevalence ranges between studies it is estimated that between 85-95% of low back pain cases can be diagnosed as mechanical low back of leg pain (LBP). Some examples of this of mechanical LBP include: lumbar strain/sprain, degenerative disc disease, spondylolisthesis, spinal stenosis, disc herniation, traumatic fracture, congenital disease (scoliosis) and internal disc disruption (that is not an exhaustive list either). Sometimes these conditions are further separated into specific and non-specific causes. The remaining 3-5% can be attributed to visceral disease (e.g. prostitis, endometriosis, nephrolithiasis, pyelonephritis, aortic aneurysm, cholecystitis, and pancreatitis) and non mechanical spinal conditions (cancer, infection, Paget disease, Scheuermann disesase and inflammatory diseases)(Jarvik & Deyo, 2002, p. 587).
As we learnt in the previous blog, within the class of inflammatory disorders there is a subgroup called seronegative spondyloarthropathies which include HLA-B27 positive conditions such as psoriatic arthritis, ankylosing spondylitis, reactive arthritis, inflammatory bowel disease and undifferentiated arthritis (Zochling & Smith., 2010).
For all of these conditions inflammatory low back pain is a clinical feature, which has 5 key features (Sieper et al., 2008):
Pain onset younger that 40 years of age.
Insidious onset.
Pain improvement with exercise.
Pain worsening with rest.
Pain at night (without improvement on getting up).
The diagnosis of inflammatory LBP is 77% sensitive and 91.7% specific if at least 4 or the 5 features are present (Sieper et al., 2008, p. 786). According to the European Spondyloarthropathy Study Group (ESSG) Classification, the diagnosis of spondyloarthropathy can be made if someone presents with inflammatory spinal pain and any of the following:
- Positive family history
- Psoriasis
- Inflammatory bowel disease
- Acute diarrhoea, urethritis or cervicitis preceding the onset of arthritis
- Alternating buttock pain
- Enthesopathy
- Radiological sacroiliitis (Zochling & Smith, 2010).
There are several clinical assessment tools I'd also like to re-mention (which are explained in further detail in part 1 of this blog): CASPAR (psoriatic arthritis), New York Classification Criteria (sacroiliitis), the ESSG (diagnosing spondyloarthropathies) and the International Classification Criteria by the ASAS (splitting symptoms into axial and peripheral).
So now that we have recapped on the key ideas presented in part 1 of this topic, let's focus on the finer details of assessment, discuss the role of medical imaging and drugs, and talk about the evidence surrounding management for these conditions.
QUESTIONING FOR INFLAMMATORY LOW BACK PAIN
Clinicians may question for inflammatory conditions when patients present with: multiple joint pains, atraumatic swollen and painful joints, more prominent morning stiffness, and night pain. If you're trying to gather more information about this potential inflammatory pain, below are some questions that go into further detail (Goodman & Fuller, 2014; Maitland, Hengeveld, Banks, & English, 2005):
- How did the pain develop? Is there a moment-in-time or history of trauma? Did it begin gradually? Were there any other changes in your life or physical health during that time?
- Do you suffer from stiffness in the morning? If so, how long does it take to settle and what do you do to ease the stiffness?
- Do you become stiff after sitting for prolonged periods? If so, how long does it take to settle after standing up?
- Do you take anti-inflammatory medication? If so, what type, dosage and effect does this have?
- Do you suffer from pain in your joints? If so, which ones?
- Have you noticed any swelling or other skin changes in these regions?
- Does anyone in your family have a history of rheumatoid arthritis or other types of inflammatory disorders such as inflammatory bowel disease or Crohn’s disease.
- Do you have any other symptoms that have occurred around the same time as the pain began such as urinary retention, pain in other parts of your body, or incontinence?
- What do you normally do to help ease the pain? This is particularly important to discuss with the patient as it gives vital information about the behaviour of the pain. For example, if there is a particular position of comfort or movement that is provocative, the pain may display a mechanical nature, whereas if no movements are more provocative or settling that others, the pain may display a more inflammatory nature.
A point to note is that these questions help us to delve more deeply into the problem presented to us. They don't act as a clinical prediction rule or diagnostic criteria. Instead these questions help you develop a hypothesis list of what the potential causes are, so that you can direct your treatment towards identifying the main contributing factor. Just remember, that in the initial consultation, you're trying to work out if the person in front of you is suitable for physiotherapy and trying to decide if they came to the best practitioner for their problem?
CONSIDERATIONS FOR ASSESSMENT
Your evaluation of this patient may need to be more detailed. Remembering that the common symptoms accompanying low back pain are enthesopathy, dactylitis, sacroiliitis, uveitis and inflammatory bowel disease, below are some changes you might consider making in your assessment:
- Assessment of spinal range of movement in the cervical, thoracic and lumbar spine - looking at the global movement of the spine, not just one region.
- Grip strength, observation of the hand and finger joints and nail condition.
- Finger range of movement at the MCP, PIP and DIP joints.
- Wrist active and passive range of movement.
- Taking note of other changes in the affected areas such as swelling, warmth to touch, tenderness on palpation, hyperalgesia and visible deformity. Often redness and swelling can be hard to detect when the underlying joint is the main source of pain, but warmth that does not settle following mobilisation is an inflammatory sign (Maitland et al., 2005).
- Ensuring that you compare their active and passive range of movement to determine if there is a strength limitation, pain limitation or true joint restriction to range of motion.
- Functional assessment of single leg stance, squat depth, single leg squat control, ability to balance on hands and knees and extend one arm and the opposite leg, kneeling push up control, and bridge control. All the while thinking "what can they do and if I give them exercises - where will I begin?"
- If you are going to recommend exercise therapy, having the treating medical practitioner do a general health check is a good idea as these patients are often at higher risk of developing cardiovascular disease.
- Asking the patient to complete pain, functional and quality of life outcome measures to gather a range of measures for re-evaluation at a later time .
Overall you are trying to gain a measure of pain, physical function, spinal stiffness, a measure for other effected joints and a global measure of quality of life. From there you can further design the most tailored and suitable exercise program. You are also trying to decide if the patient is suitable for physical therapy, what treatments might be suitable, and what the main problems are?
ROLE OF DRUG THERAPIES
There are two aims for the treatment of spondyloarthropathies. The first is to improve functional outcomes and the second is to reduce clinical deterioration (Dougados & Baeton, 2011). The reason you will refer patients to a Rheumatologist is to ensure they are managed with the best drug therapies. Often patients with either Rheumatoid Arthritis or seronegative spondyloarthropathies will receive either a pharmaceutical drug or biologic to tackle their auto-immune response. These drugs have an immunosupressant effect. Interestingly however, even though there is a difference in definition and cause between rheumatoid arthritis and seronegative spondyloarthropathies, their medication management is often similar (Papagoras & Drosos, 2012).
NSAIDS are the "cornerstone of pharmacological intervention for ankylosing spondylitis, rapidly reducing pain and stiffness after 48–72 hours" (Dougados & Baeton., 2011, p2132). The aim of NSAIDS is to reduce pain and dysfunction in the acute phases but, NSAIDS may also be prescribed on a long-term basis to try prevent spinal deterioration.
One advance in management has been the introduction of biologics such as TNF blockade drugs. Medical treatment of Rheumatoid Arthritis may include both pharmaceutical drugs and biologics, such as anti-TNF (anti tumour necrosis factor alpha). Anti-TNF a drug manufactured through biological process rather than chemical ones (pharmaceutical drugs), and acts to interfere with cytokine function, block co-stimulation of T cells and deplete B cells. Overall, this results in an immunosuppressant effect. It has been shown to have good impacts on RA and other spondyloarthropathies, but there remain concerns that there is a increased risk of infection in the early stages of drug use, and increased risk of developing lymphoma longer term (Davies, Symmons, & Hyrich., 2014). Even though such risks exist, often there is no other choice, especially if NSAIDS have failed to reduce the pain and functional loss associated with axial or peripheral arthritis (Papagoras & Drosos, 2012).
There are more conventional drugs such as methotrexate. Methotrexate is a class of drug called disease modifying anti-rheumatic drug (DMARDs) and is commonly used to treat other inflammatory conditions such as rheumatoid arthritis. It has been proven effective in the treatment of Rheumatoid Arthritis but also has positive impacts on the extra-spinal manifestations of inflammatory diseases such as psoriasis, uveitis and inflammatory bowel disease. The effectiveness of methotrexate on reducing inflammatory low back pain remains undetermined (Dougados & Baeton, 2011). Why do we care about the drug therapy side of managing inflammatory low back pain? Because the immunosuppressant medication changes how the body will respond to manual therapy and exercise therapy.
For example, psoriatic arthritis leads to joint deformity in the wrists and hands, but there may also be scaling and changes in the skin (from psoriasis). Care needs to be taken to protect joints when exercising as well as caring for skin integrity and also knowing they may having a different healing/recovery response to exercise. Perhaps a patient with severe PsA isn't suitable for hydrotherapy due to the risk of infection from a public pool? Or, perhaps you need to carefully select what weight bearing occurs through the upper limbs and when open or closed chain exercises are most appropriate? Understanding how these treatments may effect the immune function of your patient may also impact the treatment they complete in physical therapy.
Role of Medical Imaging
The earliest axial changes with spondyloarthropathies is thought to be sacroiliitis, with cervical involvement occurring much later in life (Paparo, et al., 2014). Sacroiliitis is the hallmark symptom for these conditions and generally involves the anterior-inferior synovial aspects of the joint and postero-superior ligaments, generally affecting both SIJs symmetrically (Amrami, 2012; Paparo, et al., 2014). Things that may present on imaging are "juxtaarticular osteoporosis, superficial erosions and progressive subchondral osteosclerosis" (Paparo, et al., 2014, p. 157). More severe cases lead to bone resorption and an increase in joint space.
When it comes to medical imaging, XRAY remains the most cost effective treatment option (Mattar, Salonen, & Inman., 2013). It is MRI, however, that is considered to be the current gold standard for medical imaging. Many studies are now highlighting the benefit of using MRI for early detection of joint changes based on the ability to have a T1- and T2-weighted image (Amrami, 2012; Papagoras & Drosos, 2012; Paparo, et al., 2014). MRI has the ability to show bone oedema with hypo-intensity on T1- and hyper-intensity on T2-weighted images (Paparo, et al., 2014).
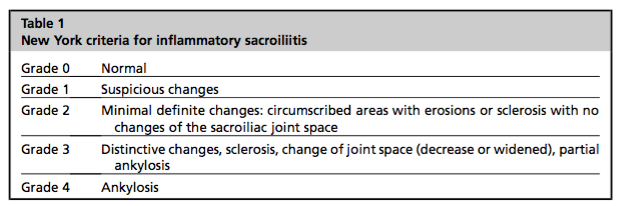
As you may have noticed, many of the diagnostic criteria use the New York Classification Criteria for sacroiliitis, despite MRI being the current gold standard. This criteria uses XRAY to rate the degree of sacroiliitis (Mattar, Salonen, & Inman., 2013, p. 652).
Unfortunately with these conditions, it can be a very long time before bone damage is evidence on XRAY, hence the push for the use of MRI in earlier diagnosis. Some authors even use the term pre-radiological axial spondyloarthropathy to emphasise the early detection of changes ((Papagoras & Drosos, 2012). CT scans also show great detail in bone lesions. I love this image to demonstrate the difference between XRAY and CT for someone with Gr 3 sacroiliitis according to the New York Classification Criteria (Paparo, et al., p. 158).
IMPLICATIONS FOR PHYSICAL THERAPY
Even though there have been developments in earlier diagnosis and better differentiation between conditions, understanding the prognosis and long term manifestations of these conditions remains to be fully understood. There have been some great advances in knowledge of these conditions over the past 10-20 years but further research into the role of physiotherapy is still required. In 2004 a systematic review was published in Cochrane (Dagfinrud, Hagen & Kvien) which concluded that there was moderate evidence to support the positive impacts of a supervised-exercise program.
From this Cochrane review the following points can be made:
- Physiotherapy intervention is better than no intervention.
- Supervised exercise groups are better than home-based exercise programs.
- Due to the poor descriptions of exercise programs in the studies reviewed, clear directions on what type of program is best can't be given.
- According to this review - "we still don't know which particular treatment protocol should be recommended" (Dagfinrud, Hagen, & Kvien., 2004).
- No RCT comparing physiotherapy interventions to other forms of exercise were found.
- Evidence to support hands-on treatments are still lacking.
- Further research is required to understand the best exercise method or intervention with specific dosage and frequency.
Oh dear! That doesn't seem too helpful? But really what it is saying is that while we still don't know exactly what the best protocol is, we still know that Physiotherapy intervention has a positive impact. From the reading I've done, exercise is definitely more researched that hands-on treatments. Perhaps this reflects the ability for exercise therapy to better address the goals of reduced pain, reduced spinal stiffness and improved functional outcomes?
One important thing to consider is that these conditions change the structural integrity of the bones and joints. On a structural level there are changes occurring in the joints that need to be considered, such as calcium depositions in the joints, destruction of collagen and reduced soft tissue tensile strength. One criticism of manual therapy that I often read about in the literature, is the difficulty to accurately measure how much load we transfer to a patient with manual therapy techniques. Normally, my response to this query is that I use the patient, my tactile sensation and clinical reasoning to determine what is a suitable grade of treatment even though I can't quantify the load in terms of kilograms or pounds. But, what if the structural integrity of you patient isn't the same as a normal joint? It definitely is a point to ponder over and probably the biggest reason why manipulation is often contraindicated in patients with acute infection or acute inflammation? It doesn't mean that manual therapy is off the table as a treatment, but just keep it in the back of your mind that it is hard to determine how much load the tissues can tolerate and how much load you are providing with your treatment. While there is some evidence on the topic of manual therapy for osteoarthritis, the studies surrounding manual therapy for this spectrum of patients is scarce.
TAKE HOME MESSAGES
The reason I wanted to write this blog is to share with you how learning more about these medical conditions can change how we conceptualise our physiotherapy approach. Admittedly, this is a difficult topic to write about and diagnosing patients with a seronegative spondyloarthropathy is by no means easy. Firstly, these patients represent a very small percentage of the population that suffers from back pain. Secondly, these conditions can be thought of as a spectrum of disorders - even though different diagnostic criteria exist, it can be challenging to distinctly separate them from each other. And finally, the symptoms might be difficult to differentiate in order to identify the primary problem. The reason I wrote this blog however, is to remind us all that the outliers do exist and every now and then our clinical reasoning will be put to the test. So the take home message is:
- Make the pieces fit.
- Be thorough with your questioning about pain behaviour and nature.
- Be thorough about your questioning of family history, medical history and other physical symptoms.
- When in doubt - ask for help.
These conditions don't exclude physiotherapy interventions, in fact, many of them are treated with a tailored exercise program. Knowing how each condition interacts with the musculoskeletal system and contributes to pain presentations will help you make your treatment more patient-centred. If you are suspicious of an inflammatory disorder then referral to a Rheumatologist would be recommended and you can support this referral by listing the information you've gathered from your assessment.
Sian
References:
Akgul, O., & Ozgocmen, S. (2011). Classification criteria for spondyloarthropathies. World J Orthop, 2(12), 107-15.
Amrami, K. K. (2012). Imaging of the seronegative spondyloarthopathies.Radiologic Clinics of North America, 50(4), 841-854.
Brosseau, L., Wells, G. A., Tugwell, P., Egan, M., Dubouloz, C.-J., Casimiro, L., . . . Bell, M. (2004). Ottawa Panel evidence-based clinical practice guidelines for therapeutic exercises in the management of rheumatoid arthritis in adults. Physical Therapy, 84(10), 934-972.
Davies, R., Symmons, D. P., & Hyrich, K. L. (2014). Biologics registers in rheumatoid arthritis. Medicine, 42(5), 262-265.
Dagfinrud, H., & Hagen, K. (2004). Physiotherapy interventions for ankylosing spondylitis. The Cochrane Library.
Dougados, M., & Baeten, D. (2011). Spondyloarthritis. The Lancet,377(9783), 2127-2137.
Ehrenfeld, M. (2012). Spondyloarthropathies. Best Practice & Research Clinical Rheumatology, 26(1), 135-145.
Gionchetti, P., Calabrese, C., & Rizzello, F. (2015). Inflammatory Bowel Diseases and Spondyloarthropathies. The Journal of Rheumatology, 93, 21-23.
Goodman, C. C., & Fuller, K. S. (2014). Pathology: implications for the physical therapist: Elsevier Health Sciences.
Gottlieb, A., Korman, N., Gordon, K., Feldman, S., Lebwohl, M., Koo, J. Y. M., . . . Menter, A. (2008). Guidelines of care for the management of psoriasis and psoriatic arthritis. Journal of the American Academy of Dermatology, 58(5), 851-864.
Mattar, M., Salonen, D., & Inman, R. D. (2013). Imaging of spondyloarthropathies. Rheumatic Disease Clinics of North America, 39(3), 645-667.
Jadon, D. R., & McHugh, N. J. (2014). Other seronegative spondyloarthropathies. Medicine, 42(5), 257-261.
Khan, M. A. (2002). Update on spondyloarthropathies. Annals of Internal Medicine, 136(12), 896-907.
Linden, S. V. D., Valkenburg, H. A., & Cats, A. (1984). Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis & Rheumatism, 27(4), 361-368.
Maitland, G. D., Hengeveld, E., Banks, K., & English, K. (2005). Maitland's vertebral manipulation (Vol. 1): Butterworth-Heinemann.
Menter, A., Gottlieb, A., Feldman, S., Van Voorhees, A., Leonardi, C., Gordon, K., . . . Bhushan, R. (2008). Guidelines of care for the management of psoriasis and psoriatic arthritis. Journal of the American Academy of Dermatology, 58(5), 826-850.
Paparo, F., Revelli, M., Semprini, A., Camellino, D., Garlaschi, A., Cimmino, M. A., ... & Leone, A. (2014). Seronegative spondyloarthropathies: what radiologists should know. La radiologia medica, 119(3), 156-163.
Papagoras, C., & Drosos, A. A. (2012). Seronegative spondyloarthropathies: Evolving concepts regarding diagnosis and treatment. Journal of Spine,2012.
Rudwaleit, M., Van Der Heijde, D., Landewé, R., Akkoc, N., Brandt, J., Chou, C. T., ... & Van den Bosch, F. (2010). The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Annals of the rheumatic diseases, annrheumdis133645.
Sieper, J., van der Heijde, D. M. F. M., Landewe, R., Brandt, J., Burgos-Vagas, R., Collantes-Estevez, E., ... & Van Der Linden, S. (2009). New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Annals of the rheumatic diseases, 68(6), 784-788.
Taylor, W., Gladman, D., Helliwell, P., Marchesoni, A., Mease, P., & Mielants, H. (2006). Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis & rheumatism, 54(8), 2665-2673.
Zochling, J., & Smith, E. U. (2010). Seronegative spondyloarthritis. Best Practice & Research Clinical Rheumatology, 24(6), 747-756.